The research group of Lendület Neuroimmunology at ELKH Institute of Experimental Medicine (IEM), led by Ádám Dénes, is the first to identify the role of microglial cells in the regulation of cerebral blood flow and neurovascular coupling. Their work was published in the prestigious international publication entitled Journal of Experimental Medicine. Their results are also crucial for understanding the mechanisms of brain perfusion deficits that often underlie common neurological diseases (such as stroke, Alzheimer's, Parkinson's, epilepsy and dementia).
Microglia are primarily recognized by the scientific community as the most important immunocompetent cell type in the brain and as the main regulator of brain inflammatory processes. Its altered activity is known to play a role in the development of diseases such as stroke, epilepsy, Alzheimer's disease, Parkinson's disease and ALS. The investigation of the physiological and pathological role of microglia is a popular and intensively developing field of research. As a result, a substantial body of knowledge has been accumulated in the field of microglia-neuron interactions. The Neuroimmunology group itself has several internationally recognized publications in this regard. In contrast, little is known about the relationship of microglia to blood vessels and cerebral blood flow, although microglial cells are very early sensors of inflammatory changes or damage to blood vessels and have been hypothesized to play an active, supporting role in some vascular processes.
The research program, carried out by the research group of Ádám Dénes with outstanding contributions from Eszter Császár and Nikolett Lénárt, has demonstrated the essential role of microglia in neurovascular coupling, hypercapnia-induced vasodilation and adaptation to cerebral hypoperfusion, using a multifaceted approach, a variety of in vivo imaging and non-invasive methods, high-resolution molecular anatomy techniques and in vitro imaging. These mechanisms operate via specific, dynamically changing anatomical relationships between microglia, neurons and cells of the neurovascular unit involved in the regulation of cerebral blood flow, which the researchers have identified in this paper. The significance of this discovery is illustrated by the fact that altered microglial activity, reduced cerebral blood flow and impaired neurovascular coupling response precede the development of neurological symptoms in common neurological diseases such as Alzheimer's disease, other forms of dementia and stroke.
The research team's findings have also demonstrated that dysfunction of microglial cells modulates cerebral blood flow by altering their complex interactions with cells in the neurovascular unit. The discovery may have important clinical implications. Among others, altered microglial activity may influence clinical outcome in dementia, stroke or after transient ischemic attack (TIA) through modulation of cerebral blood flow or adaptation to reduced perfusion, especially in patient groups with risk factors for carotid stenosis, aneurysm, hypertension or chronic vascular inflammation. Acting via similar cellular interactions, microglia may also contribute to circulatory failure following cerebral ischaemia or vasospasm due to subarachnoid haemorrhage. Thus, microglia should be considered an important modulatory cell type in physiological and pathological changes of cerebral blood flow. Understanding these mechanisms may facilitate the development of therapies for a number of neurological diseases.
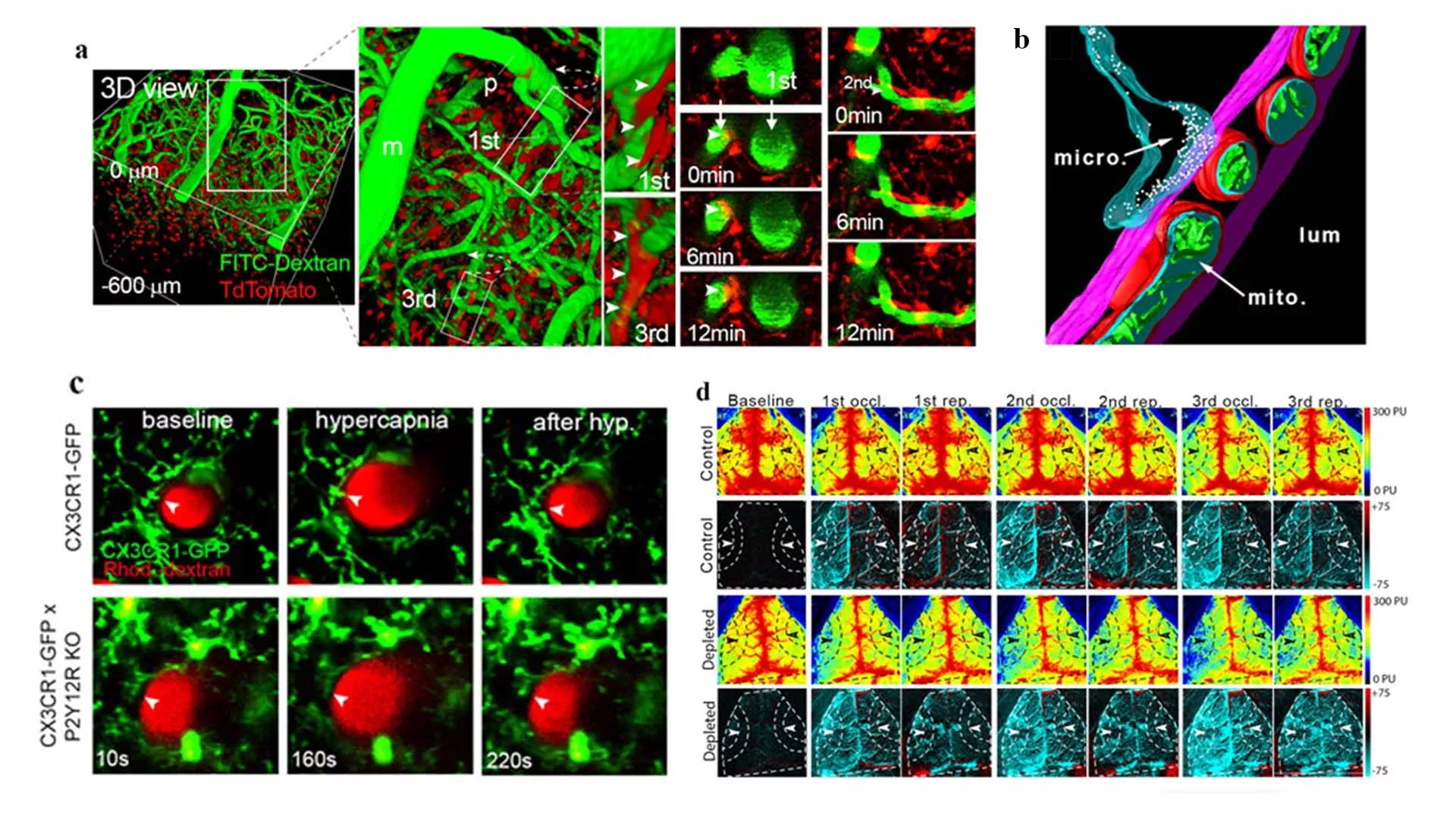
The study was co-first authored by Eszter Császár and Nikolett Lénárt, and published in the Journal of Experimental Medicine. DOI: 10.1084/jem.20211071
The research was supported by the following research grants received by the Laboratory:
ERC Consolidator Grant (ERC-CoG 724994) of The European Research Council, Lendület Programme (LP2016-4/2016) of the Hungarian Academy of Sciences, The Hungarian Brain Research Programme (KTIA_13_NAP-A-I/2). The project has also received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie "ENTRAIN" grant (No. 813294). Nikolett Lénárt, Csaba Cserép and Krisztián Szigeti were awarded a János Bolyai Research Fellowship from the Hungarian Academy of Sciences. Csaba Cserép (UNKP-20-5), Balázs Pósfai (UNKP-20-3-II) and Nikolett Lénárt (UNKP-21-5) also received a grant from the New National Excellence Program of the Ministry for Innovation and Technology.