Team leaders of the ELKH Institute of Experimental Medicine Budapest, Drs. Balázs Gereben and Csaba Fekete, developed an innovative research tool to study cell-type-specific thyroid hormone (TH) signalling. Their patented mouse model recently allowed to achieve groundbreaking results published in eLife with translational consequences for the treatment of hypothyroidism.
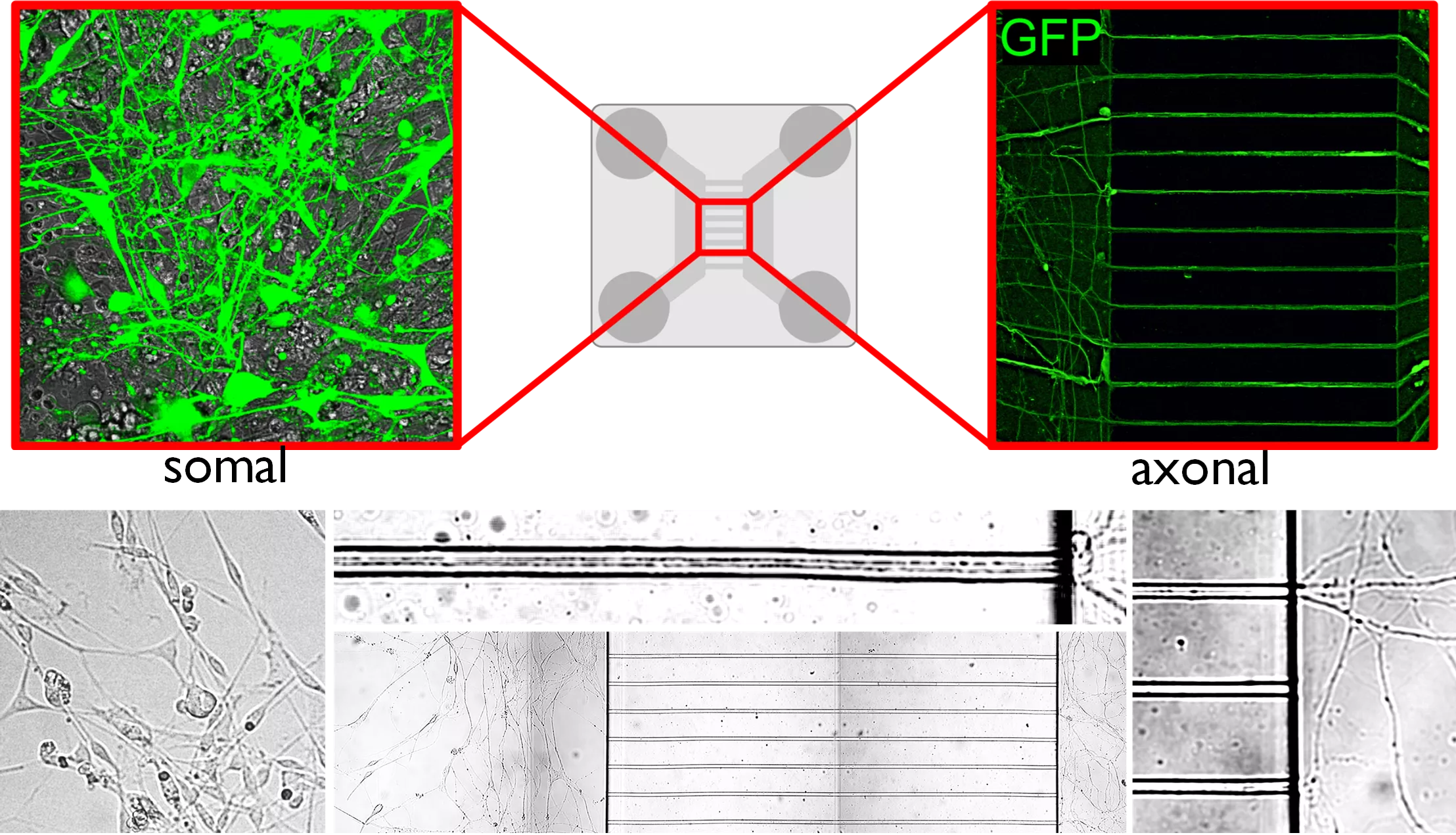
Figure 1. Bicompartmental microfluid primary cultures of neurons for T3 trafficking studies
Axons growth through microcapillaries and allow trafficking studies based on a functional separation of the cell body and the axonal ending of the same cell
TH is a master regulator of cellular function in virtually all tissues and plays a major role in the regulation of brain function. The circulating level of TH in the bloodstream is relatively stable, which is in striking contrast to the turbulent and quick changes of TH levels in tissues, which is required to allow tissue-specific TH signalling to meet the locally and timely changing needs of TH availability in various tissues and cell types.
TH economy of the brain is especially complex since this organ is both the regulator and target of TH. Up to now, it was an unresolved question, how T3, the activated form of TH reaches its targets inside the brain to evoke its biological effect. It was predominantly thought that it has a paracrine effect, thus glial cells generating T3 from its prohormone T4 supply the hormone to neighbouring neurons.
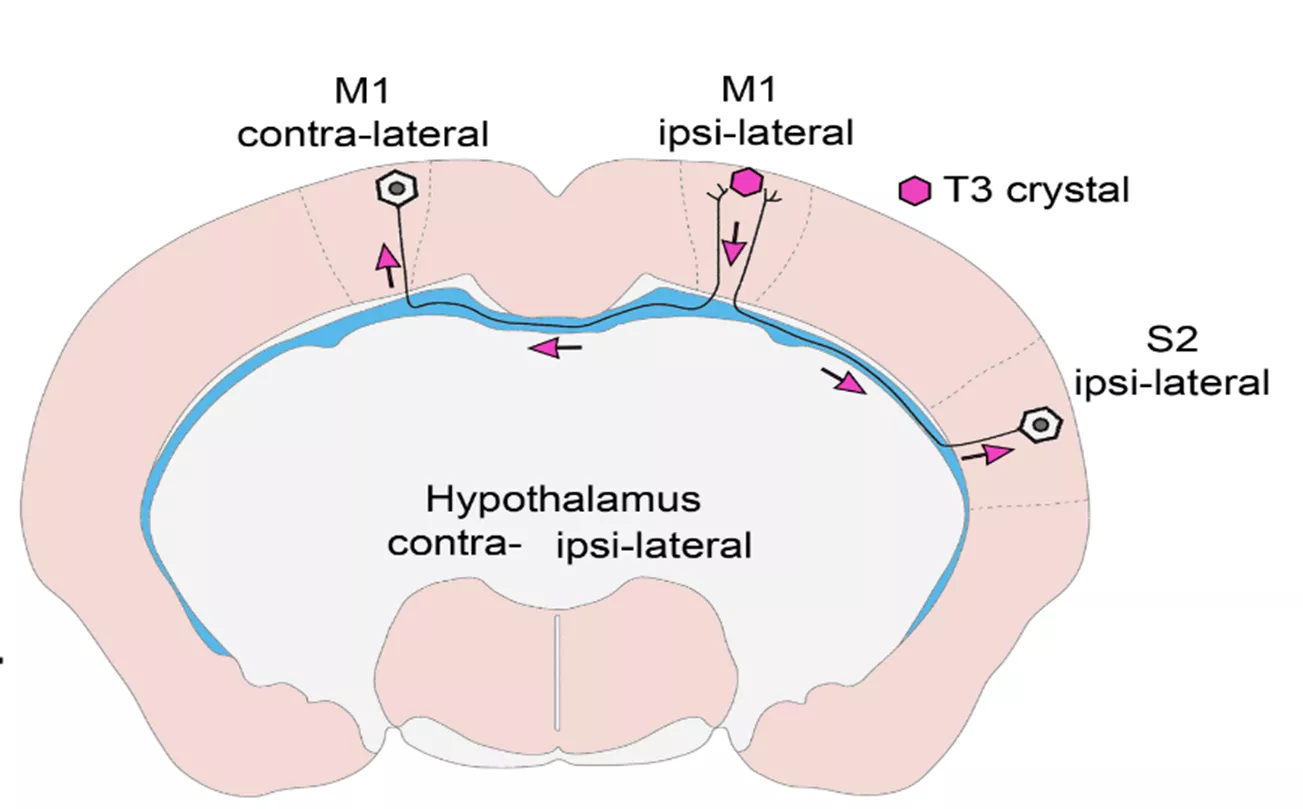
Figure 2. Studies of T3 trafficking in the brain of the Thyroid Hormone Action Indicator mouse. T3 was placed into the M1 cortical region of THAI mice, and it travelled to the other hemisphere via interhemispheric axons and evoked transcriptional effect in the contralateral region while did not show up in the unconnected hypothalamus.
In a complex set of in vitro and in vivo experiments, the researchers were able to prove that T3 travels retrogradely in neuronal axonal processes to the nucleus of these cells and the journey occurs in vesicles that keep the hormone protected from the high level of T3 degrading enzyme in neurons. They showed that TH signalling in the brain is far more complex than earlier thought: T3 generated in the cerebral cortex can travel to the other cortical hemisphere via long interhemispheric axons and consequently, distantly located brain regions can regulate each other’s TH-mediated cellular activities. In addition, these studies also answered the long unresolved question, how the neuronal cell group that regulates the hypothalamic-pituitary-thyroid (HPT) axis, the hypophysiotropic TRH neurons of the hypothalamus, receive the T3 input from the peripheral circulation and from tanycytes, the TH-activating cells of the hypothalamus. This has consequences on the whole-body level since the TH-evoked negative feedback regulation of TRH neurons is a crucial regulatory mechanism of the HPT axis. Finally, the obtained results have translational consequences for the treatment of hypothyroidism since they explain why the T3 component of the T4+T3 combinational therapy can survive T3 degrading activity of the brain and can be beneficial for specific TH-supplemented patients who do not respond well to T4 monotherapy.
The unique opportunity to study T3 trafficking in the brain was provided by the Hungarian, EU, and US patent-possessing Thyroid Hormone Action Indicator (THAI) transgenic mouse model developed by the common effort of Balázs Gereben and Csaba Fekete research groups.
The present project was founded by the US National Institute of Health (NIDDK R01DK058538) and was performed in collaboration with the group of Dr. AC Bianco at the University of Chicago. The paper was published in the prestigious journal eLife. Both the first and last authorship were shared by the Hungarian and US research groups.
Publication:
Federico Salas-Lucia, Csaba Fekete, Richárd Sinkó, Péter Egri, Kristóf Rada, Yvette Ruska, Balázs Gereben, Antonio C Bianco (2023). Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain. eLife, May 19, 2023. DOI: 10.7554/eLife.82683