The HUN-REN-ELTE Protein Modelling Research Group led by András Perczel observed that amyloids are not only involved in the development of neurodegenerative diseases, but also in beneficial physiological processes such as blood sugar regulation. The discovery has led to an understanding of the specific spatial structural properties of certain hormone proteins that, when altered by the environment, influence processes throughout our body. The study, which presents the results, has been published in the prestigious journal Nature Communicatons.
In our ageing society, the management of neurodegenerative diseases is a major social and scientific challenge, which, in addition to the healthcare system, also poses new challenges in the field of research, development and innovation. This group of diseases, such as Alzheimer's and Parkinson's, is characterised by the abnormal "deposition" of proteins, forming so-called amyloid tangles, which cause the destruction of cells and tissues such as nerve cells, the heart muscle or the lungs. At the molecular level, it is the formation of amyloid tangles that underlies these abnormal and sometimes fatal transformations. This is the inappropriate folding, or misfolding of certain functional proteins by some effect, which then leads to unwanted precipitation, amyloid formation and aggregation. However, the physiological processes and the molecular basis of amyloidosis and neurodegenerative diseases are still far from being understood.
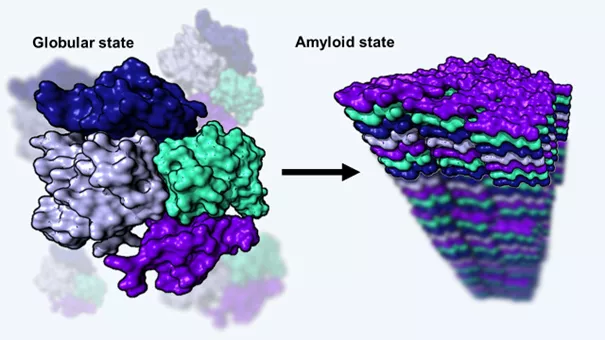
Functional (left) and pathological (right) folds of the human transthyretin protein: the latter amyloid form is deposited in various tissues of the body and leads to health damage.
The study of the amyloid state remains a major challenge today, but is essential for effective drug development against neurodegenerative diseases. Thanks to advances in modern techniques used by structural chemists and biochemists at ELTE, including cryo-electron microscopy, nuclear magnetic resonance (NMR) spectroscopy and X-ray diffraction, researchers can now image not only correctly folded proteins but also misfolded or even unfolded proteins at atomic resolution, allowing them to investigate the molecular interactions between healthy and pathological proteins. High-resolution structure determination and imaging allows the ELTE Laboratory of Structural Chemistry and Biology - which was ranked among the Top 50 laboratories in Hungary - to understand and scientifically investigate the normal and abnormal 3D-structure of proteins.
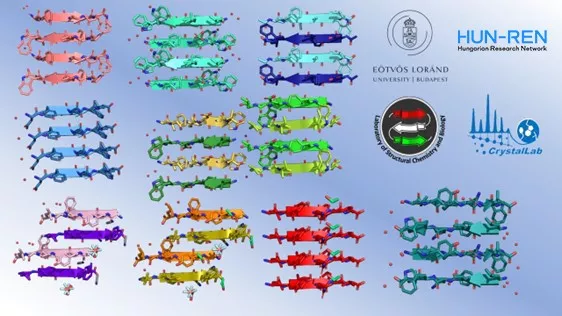
A schematic drawing of the amyloid-structure patterns of the hormone fragments determined by X-ray crystallography by Zsolt Dürvanger, a member of the research team.
Some polypeptides and proteins are not only present in the so-called bioactive globular form in the body, but also exist intermittently in a fibrous amyloid form. These are functional amyloids. In this case, the amyloid storage form is not associated with any disease, and in fact plays a physiologically important role. Such shorter proteins include, for example, certain hormones responsible for blood glucose regulation and digestive health, such as glucagon or glucagon-like peptides (GLP-1 and GLP-2). These hormones are stored in a pH-dependent amyloid form after biosynthesis and then, upon stimulation, lose their amyloid character when released into the bloodstream and exert their beneficial physiological effects in a globular form.
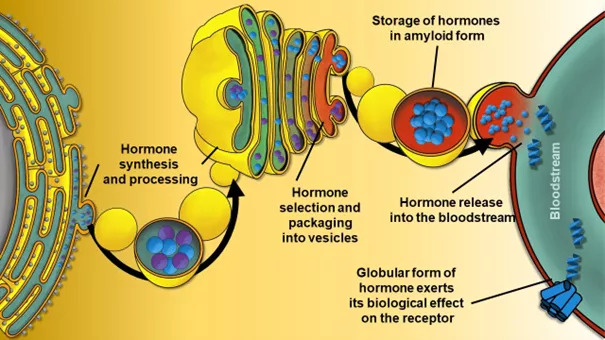
Synthesis, processing and storage of hormone proteins in "beneficial" or functional amyloid form, and finally delivery to the target receptor after breakdown of the amyloid fibres, schematic diagram according to first author Dániel Horváth.
The latest research results of the HUN-REN-ELTE Protein Modelling Research Group led by András Perczel, published in Nature Communications, have now led to an understanding of the structural properties of these hormone proteins that allow the same protein to exist in two forms, i.e. it is stored in amyloid form and then released to act one by one as monomers on cell surface receptors and regulate the whole body.
The aim of the research is to better understand the structural factors that can cause amyloid-like protein deposits in proteins. The results suggest that an ancient, evolutionarily conserved amino acid sequence in hormones - a molecular sequence that is found in all vertebrate species - is primarily responsible for receptor binding, but also catalyses the folding of molecules into amyloid-like fibres. The amino acids in this segment - glutamic acid and aspartic acid - ensure that the molecules are either stuck together or split apart, depending on the acidity of the environment. This pH-dependent molecular switch or coupling allows the amyloid form to be taken up (assembly) and then the globular form to be formed (disassembly or monomerisation), depending on the actual chemical environment.
Glucagon-like peptides, the focus of this study, are now in the spotlight because their synthetic derivatives can be used effectively in the treatment of type 2 diabetes. In fact, these molecules are the first clinically effective weight-loss drugs that also have a beneficial effect on our cardiovascular system. Based on these findings, the researchers stress that the use of the beneficial functional amyloid form of these medically important hormones will allow the development of products with a long-acting profile, making it easier for patients to administer. The new cryo-electron microscopy centre currently under construction at the University of Pécs, which is expected to be operational in 2024, will also be of great importance for the success of these projects.
Publication:
Horváth, D., Dürvanger, Z., K. Menyhárd, D. et al. Polymorphic amyloid nanostructures of hormone peptides involved in glucose homeostasis display reversible amyloid formation. Nat Commun 14, 4621 (2023). DOI: 10.1038/s41467-023-40294-x