In March 2020, at the start of the spread of the COVID-19 epidemic in Hungary, a consortium including researchers from the ELKH Research Centre for Natural Sciences (RCNS) began developing and investigating the antiviral preparation of favipiravir in Hungary. Previous Japanese studies had shown that tablets containing favipiravir prevent the virus from multiplying in the early stages of the disease, indicating that its use may play a key role in controlling the epidemic. Miklós György Keserű, senior researcher at RCNS, has reported on the process of developing medication in Hungary containing favipiravir as an active ingredient.
The development of favipiravir, carried out with the support of the Ministry of Innovation and Technology, has several key objectives. These include the continuous provision of care to Hungarian patients, the ramp-up of production through development and export, and the demonstration of the efficacy and safety of favipiravir in tackling COVID-19 infection through scientific clinical trials.
Imported medications that contain favipiravir are currently successfully being used as an off-label treatment for Hungarian coronavirus patients with OGYÉI (Hungarian National Institute of Pharmacy and Food Health) approval. This option is included in the Hungarian Coronavirus Handbook, and experience has demonstrated that the drug can be used effectively and safely in the initial viral stages of a coronavirus infection to treat the infection and prevent the development of more serious complications.
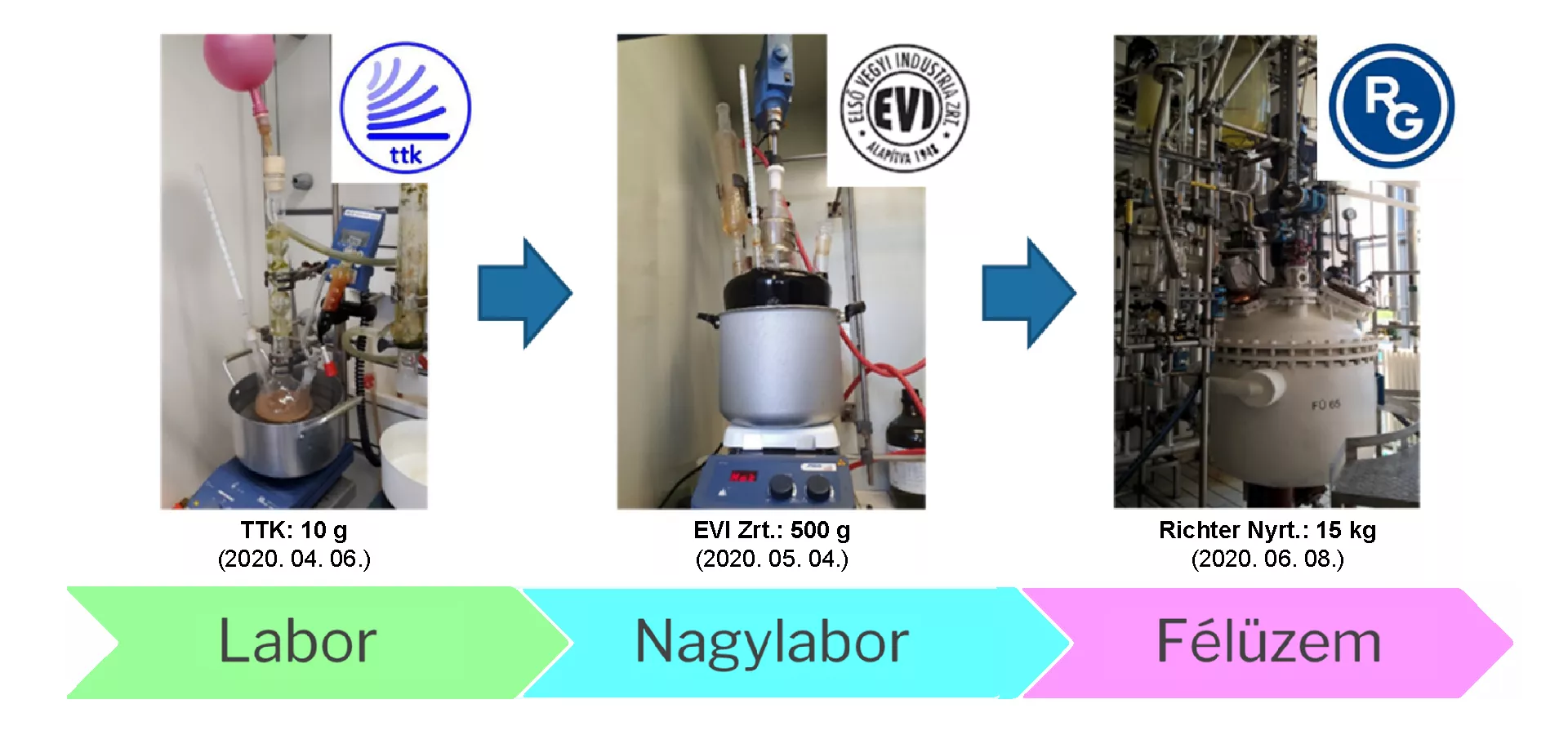
The researcher said that several methods have been developed for the preparation of medicines containing favipiravir, which means the fundamental structures were already in place. However, he also added that these formulations often do not contain all the important details and are mostly not designed for pilot or factory-scale syntheses.
Although it is a relatively simple molecule, the development of favipiravir entails numerous challenges. Although there are many different ways to produce favipiravir medication, the researchers had to identify the best of the possibilities in the shortest possible time, i.e. the one that could be used quickly and economically to produce the right quality and quantity of the active ingredient. To do this, it was necessary to perform a multitude of laboratory experiments. The development of a suitable synthetic route is inconceivable without a detailed examination of the quality of the initial materials, intermediates and, of course, the final product. The development was also hampered by the fact that for quality control purposes, analytical methods had to be developed in parallel with the synthesis. The RCNS Instrumentation Centre played a key role in the development of the test methods. An additional problem for researchers was that due to the huge demand, almost all of the raw materials for favipiravir were in short supply, and due to transport capacity constraints, procurement was also a lengthy process. As a result, the members of the consortium responsible for production turned to the logistics team of the Ministry of Foreign Affairs and Trade, with the help of which they were able to procure the raw materials and produce the active ingredient by the beginning of the second wave.
A number of noteworthy collaborations have taken place as part of the development consortium. Synthesis in a large laboratory was carried out in the laboratory of Első Vegyi Industria Zrt., pilot production at Richter Gedeon Nyrt., and production of the pharmaceutical product at Meditop Kft. As a result of the joint efforts of the consortium partners, Hungary now has an efficient and reliable process for the production of medicine containing favipiravir. The development of the technology has been successfully completed and the establishment of the legal conditions for production and distribution are currently in progress.
In the meantime, preparations for a clinical trial of the medicine are already under way. With the permission of the National Institute of Pharmacy and Food Health, the study will be performed by a consortium specializing in HECRIN (Hungarian Clinical Research Infrastructure Network) clinical trials led by the University of Pécs.