Research groups from the HUN-REN Research Centre for Natural Sciences (HUN-REN TTK), in collaboration with colleagues from the Center for Cancer Research at the Medical University of Vienna, identified a targetable novel mechanism within tumor cells that survive chemotherapy. The findings could represent a step forward in the treatment of treatment resistant cancer. The paper presenting their discovery was published in Drug Resistance Updates, a highly prestigious journal of drug resistance research.
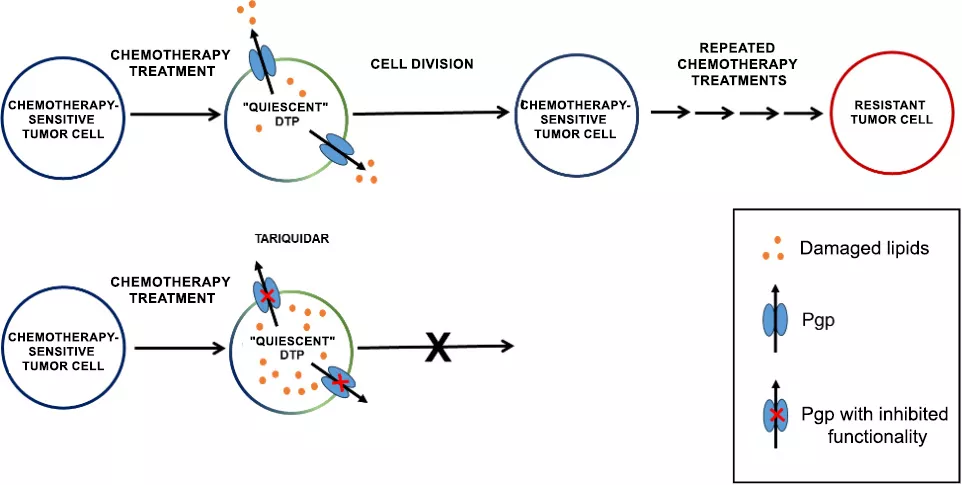
Eliminating chemotherapy-surviving drug-tolerant persister (DTP) cells by inhibiting the Pgp transporter.
Resistance in focus
Successful treatment of tumors is still a significant clinical challenge. Some cancer patients respond exceptionally well to initial therapies: the tumor size decreases, and it may even completely disappear, accompanied by an improvement in overall well-being. Unfortunately, there are instances when the tumor reappears and becomes resistant to further treatments over time. Over the past decades, research has focused on understanding the mechanisms of therapy resistance and targeting resistant tumors, with the assumption that eliminating the mechanisms that sustain resistance could lead to prolonged survival and even complete recovery for patients.
Researchers from HUN-REN TTK investigated the early stages of therapy resistance development in a new approach. Rather than focusing on already resistant cancer cells, they sought to answer the seemingly simple question: if a significant portion of tumors responds well to treatment, what explains the recurrence of tumors? It is evident that some cells survive the therapy and can remain dormant for several months or even years.
During the study of this phenomenon, the challenge lies in the rarity of cells surviving treatment. Therefore, researchers from the HUN-REN TTK Drug Resistance Research Group, the Metabolic Drug Interactions Research Group, and the Centre for Structural Sciences, in collaboration with colleagues from the Center for Cancer Research at the Medical University of Vienna, developed a new model system. In this system, following high-dose chemotherapy, the majority of breast tumor cells are destroyed, but as is the case in patients, a few cells persist. The investigations revealed that these surviving cells, known as drug-tolerant persister (DTP) cells, remain alive despite suffering severe DNA damage. The surviving cells exhibited unusual, bizarre morphology, did not divide at all, and appeared to eventually perish. To the surprise of the researchers, however, the "quiescent" cells awakened weeks later and resumed proliferation.
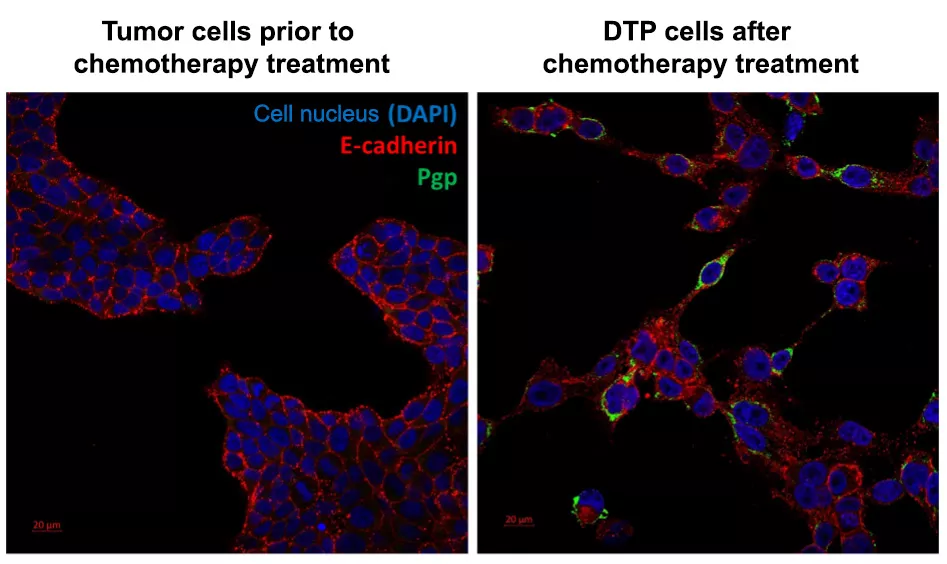
As a result of chemotherapy treatment, the E-cadherin protein, originally found in the tumor cell membrane, appears also in the cell bodies, and drug-tolerant persister (DTP) cells begin to express the Pgp transporter.
The Achilles' heel of surviving tumor cells
The tumors re-emerging from DTP cells did not become resistant, suggesting that tumor survival can be explained by transient factors rather than the selection of originally resistant cells. Through a more thorough examination, it was revealed that a fraction of the surviving cells expresses a protein called P-glycoprotein (Pgp), and that the inhibition of this protein fully prevents cell reawakening. The relevance of Pgp is well-known in resistant tumors, where its role is to recognize and expel chemotherapy drug molecules from the cells. However, dormant cells did not require this defence because chemotherapy agents were no longer present in their environment.
This discovery prompted the researchers to understand the role of Pgp in protecting DTP cells and, if possible, develop targeted therapies. The research team realized that, in addition to the direct toxic effects of chemotherapy, a secondary, prolonged consequence is also to be considered. The key to the survival of the DTP cells is their ability to cope with the long-term consequences of toxic chemotherapy.
One such threat is the accumulation of oxidized lipids, which can initiate a chain reaction that inevitably leads to cell death. Inside cells, multiple proteins are responsible for the neutralization of oxidized lipids. However, in specific cases such as during chemotherapy, the extent of damage exceeds the cells’ capacity, necessitating secondary mechanisms that can alleviate the pressure on the cell. This is when Pgp intervenes, transporting damaged lipids out of the cell, thereby providing a chance for the survival of DTP cells.
A novel application of a long-known drug
Based on laboratory observations, researchers aimed to utilize the Pgp inhibitor tariquidar not against already resistant tumor cells but targeting the persister cells surviving chemotherapy. The new therapeutic protocol was tested in a genetically engineered mouse mammary tumo model that closely models triple-negative hereditary breast cancer found in humans.
According to the model developed in the laboratory, the inhibitor was administered after chemotherapy, preventing side effects associated with the increased the toxicity of concurrently administered chemotherapeutic agents on healthy cells. The results unequivocally confirmed the in vitro models: the mice in which early survivor DTP cells were targeted with tariquidar after chemotherapy not only remained tumor-free for a significantly prolonged period but also lived longer than their counterparts.
It should be emphasized that in this work, the researchers proposed the new application of an agent that had previously met the strict requirements regulating clinical use. Since there is no need for years-long preclinical trials in this case, researchers are hopeful that, with the involvement of the right partners, the clinical testing of their idea for more effective cancer treatment can commence soon.
Publication:
Kornélia Szebényi, András Füredi, Eszter Bajtai, Sai Nagender Sama, Ágnes Csiszár, Balázs Gombos, Pál Szabó, Michael Grusch, Gergely Szakács: Effective targeting of breast cancer by the inhibition of ABCB1-mediated removal of toxic lipid peroxidation byproducts from drug tolerant présiére cells, Drug Resistance Updates, https://doi.org/10.1016/j.drup.2023.101007