The RT-qPCR testing procedure developed at the Szeged Biological Research Center is extremely sensitive, cost-effective and suitable for mass testing.
The researchers at the Institute of Genetics of the ELKH Szeged Biological Research Center (SZBK) led by Péter Vilmos, have developed the most sensitive laboratory test to date for the detection of the SARS-CoV-2 virus from nasal secretions. The cooperating partners of the research and development project are the Institute of Medical Microbiology and Immunobiology of the University of Szeged, the No.1 Department of Infectious Diseases of the Internal Medicine Clinic, headed by Katalin Burián and Edit Hajdú, and the ELKH Alfréd Rényi Institute of Mathematics. The new method uses a known procedure. However, due to their innovative enhancements the method is 50 times faster than the RT-qPCR diagnostic procedure used today, enabling reliable mass screening, potentially covering the entire population. This could open up a whole new perspective in prevention, something that is particularly timely given the expected new wave of infections from Covid-19.
The most significant feature of this method is that it can test a pooled sample of 50 people at a time in a single reaction without compromising reliability. Another important feature of the method is that it can identify all infectious individuals, including asymptomatic patients, thus allowing effective control of the spread of the pandemic. In addition, the test can be performed with less expensive, domestically available compounds, which is a major advantage due to the worldwide shortage of more expensive reagents as a result of the pandemic. In addition, the new testing protocol could be used not only to detect the SARS-CoV-2 virus, but may also be easily adapted to new pandemics in the future and to monitoring the spread of any pathogen, which will make it easier to control any future pandemic.
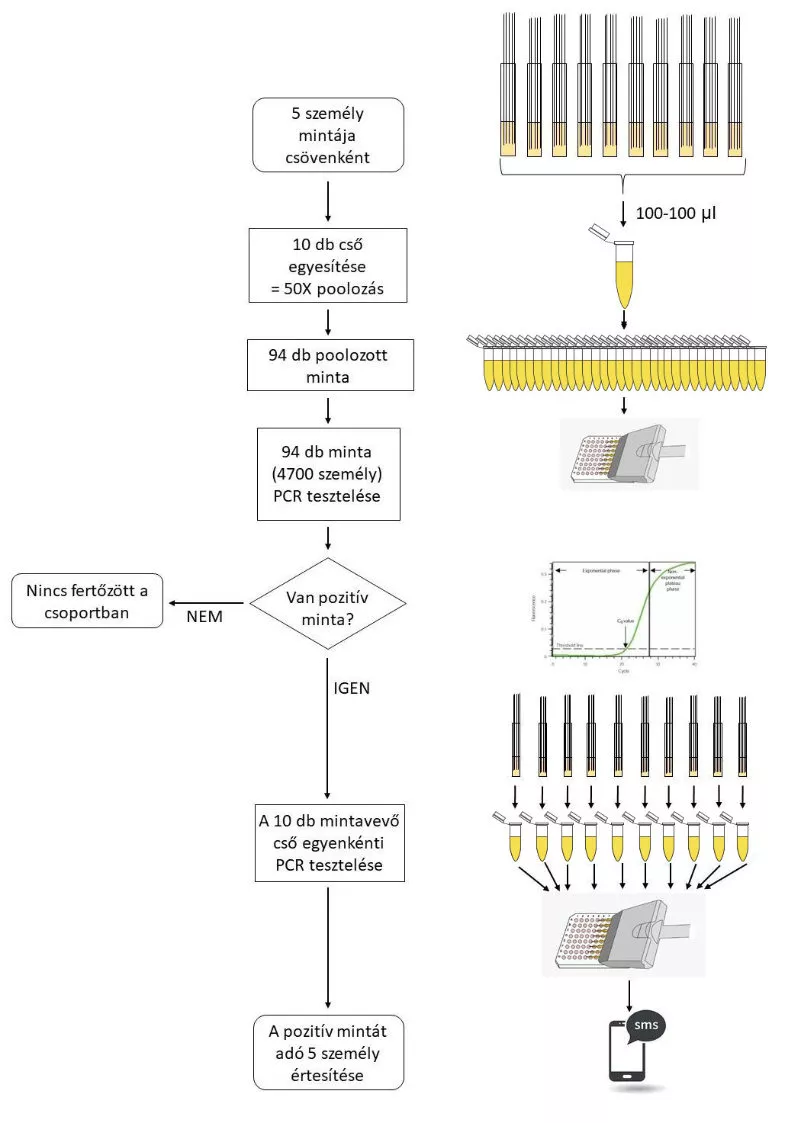
The new method is based on the extremely high-sensitivity detection of the SARS-CoV-2 virus inheritance, which enables the detection of even as low number as five pathogens, even when a large number of nasal samples are mixed. This result was made possible by several modifications to the testing procedure currently in use worldwide. The new testing procedure combines the 50 nasal samples in two steps, which ultimately leads to only a ten-fold dilution for viruses, even from a single positive nasal sample. Simultaneous detection of multiple SARS-CoV-2 genes using the simplest method available and repetition of the reaction underlying the detection significantly increases the efficiency and specificity of the new method and greatly offsets the loss of sensitivity resulting from sample pooling.
The laboratory phase of development is complete, and the method is ready to be tried in mass testing. The procedure could certainly be of considerable international interest given that the US Food and Drug Administration (FDA) said in a statement issued on June 16 that it promised to accelerate the development and recognition of new sample pooling procedures to be developed for mass testing.