Researchers at the Research Centre for Natural Sciences have developed a conceptually new photolabile linker that may further increase the localization precision of targeted chemotherapy.
Therapeutic methods that aim at selectively concentrating drugs in the proximity of tumors are considered as major achievements of modern drug research. Thanks to developments in the field of targeted drug delivery, both the therapeutic concentration of the cytotoxic drugs and the unwanted side effects are remarkably reduced.
Several strategies have emerged, which restrict the effects of the drugs in the vicinity of tumor cells. One of the approaches chemically conjugate the drugs to so-called directing vectors such as antibodies that are able to selectively bind to cancer cells. While connecting the two parts, the drug transitionally loses its biological activity. However, when it is liberated from the conjugate in the proximity of tumor cells, its cytotoxic or proliferation blocking activity reinstates. It is highly important that liberation, thus activation of the drug only occurs near the tumors in response to a chemical or physical trigger. Such chemical or physical stimuli can be the significantly different pH of tumorous tissues or light of certain wavelength. Again it should be emphasized that premature activation of the drugs compromise localization precision, thus such conjugates should be stable under physiological conditions.
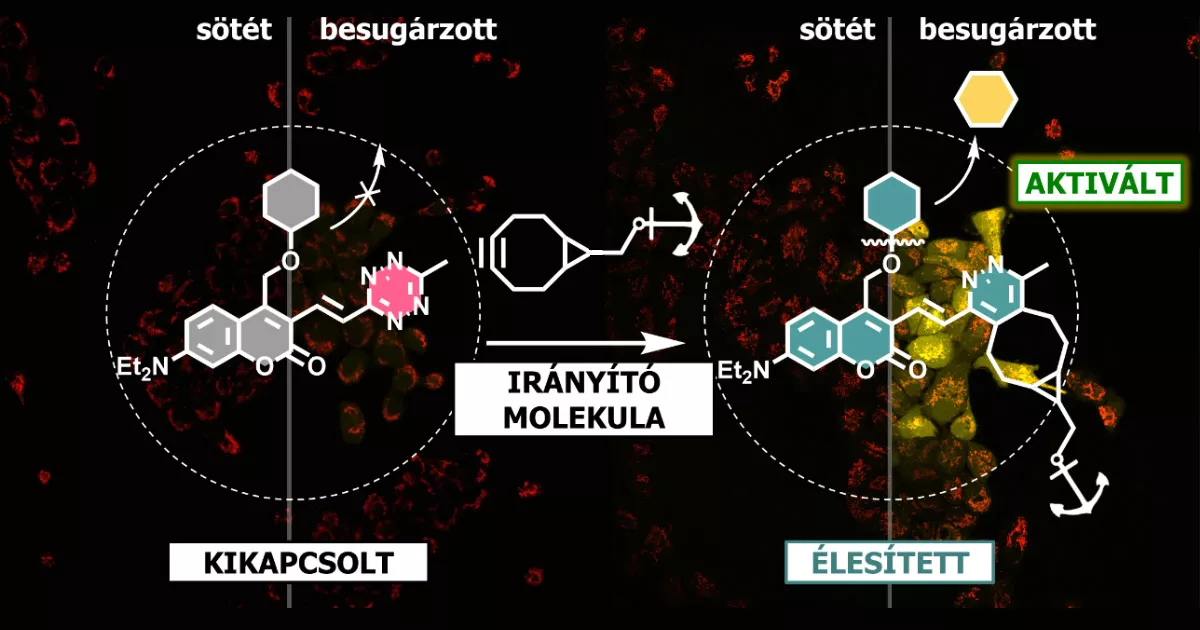
Researchers at the Chemical Biology Research Group, RCNS aim at developing photolabile compounds that can further improve the localization precision of light assisted activation of chemotherapeutic drugs. Their approach is based on the sequential addition of the targeting moiety and the inactivated drug. Due to the lagged administration of the components, the targeting moiety has already been concentrated on the surface of the target cells, when the inactivated drug reaches it. Both the targeting moiety and the inactivated drug possess functional groups that can react solely with each other under physiological conditions (such reactions are called biorthogonal). When this chemical bond is formed between the targeting moiety and the inactive drug the motif, which is responsible for the inactivation, gains and extra characteristic: it becomes photolabile. Once it happens, illumination of this construct with a suitable wavelength of light leads to liberation, thus activation of the drug. Both events, i.e., the chemical bond formation and light illumination is necessary for the drug release. This conditional photoactivation ensures that the drug is liberated uniquely in the vicinity of the targeted cells.
The work by Márton Bojtár, Krisztina Németh, Farkas Domahidy, Gergely Knorr, András Verkmann, Mihály Kállay and Péter Kele demonstrating the proof-of-concept of conditional photoactivation was published in the highly prestigious, Nature indexed1 Journal of the American Chemical Society.2 In their paper they report on the development of a multifunctional inactivator molecule that can to bind to bioorthogonally functionalized targeting moieties while becoming photolabile, thus able to release its cargo (e.g., a drug) upon illumination with a suitable laser beam. To demonstrate this new photochemical concept they used a probe instead of a drug, which becomes fluorescent when liberated from the assembled construct. Appearance of fluorescence enabled visualization of the whole process through a fluorescent microscope.
Though the path to apply the new concept in medicine is still long, present results pave the way for further experiments aiming at the translation of these fundamental research results into therapeutic use.