The staff of the Research Centre for Natural Sciences, in collaboration with their Swedish colleagues, have developed a new type of molecular switch, the operation of which is based on a different concept compared to the systems studied so far.
Molecular switches are molecules whose structure can be reversibly changed between at least two stable states by an external stimulus (e.g. light or heat). These substances are being studied in many disciplines, as they make it possible to control processes at different molecular levels by changing their structure. Their use can affect, for example, the flow of charge carriers, which is of interest from a molecular electronics point of view, or control the biological effects of the active substance of drugs in terms of location and time.
One of the most extensively studied families of molecular switches is that of dithienylethenes, which are able to switch between an “open” and a “closed” form under the influence of light of the appropriate wavelength, along with significant rearrangement of their electron systems. So far, this rearrangement has appeared to limit the range of structures that can be used as switches, as the carbon-carbon double bonds that form the “soul” of the switch are isolated, but are, at the very least, highly localized in known and functional systems. Although there may be a number of interesting applications for switches where this double bond is part of a delocalized aromatic system, such derivatives have hardly been studied at all to date. This is because, in contrast to localized double bonds, aromatic delocalization gives the molecule a stability in the ground state that has hitherto been thought to make the system resistant to rearrangement by light, i.e., it does not switch.
Péter Pál Kalapos and Gábor London, members of the Functional Organic Materials Momentum Research Group at TTK, studied the photochemical properties of the dithienylbenzene molecule containing an aromatic benzene ring in collaboration with Professor Bo Durbeej's Theoretical Chemistry Research Group at the University of Linköping. Their approach was based on the Baird rule, which says that an aromatic system becomes antiaromatic in the excited state. This anti-aromaticity in excited state is unfavorable to the molecule, which wants to exclude it in any possible way. The assumption was that switching may be a possible way to escape the unfavorable excited state.
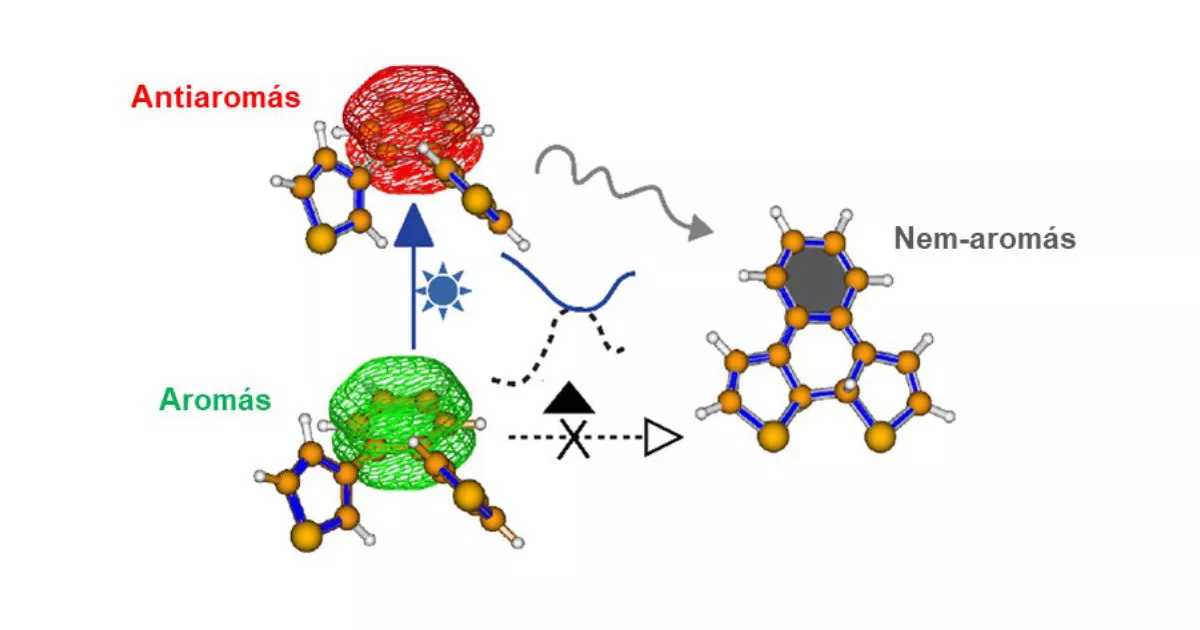
The researchers confirmed this concept with both experimental and theoretical chemical methods, thus extending the possible structures of dithienylethene-type switches to molecules containing an aromatic unit. Their results have been reported in a leading journal of chemistry, the Journal of the American Chemical Society, [1] a journal listed by the Nature Index [2].