Róbert Tuba, the head of the Institute of Materials and Environmental Chemistry research group at the ELKH Research Centre for Natural Sciences (TTK), in collaboration with his international partners, has investigated the synthesis of new-generation, macrocyclic polymers that could contribute to the development of novel, sustainable polymers with outstanding physical properties. Their results were published in the prestigious chemical journal Nature Chemistry.
Cyclic (ring) polymers are topologically special macromolecules and their physical properties differ from their linear analogues in many cases. Thanks to their ring topology these polymers do not contain chain end groups, so they also have exceptional chemical stability.
Macrocyclic polymers are used, among other purposes, as additives in lubricants. It is a natural process for the viscosity of lubricants to decrease over time: the primary reason for this is because the poly(alpha)olefins they contain undergo chain fragmentation. At the same time, opening the chain of ring polymers produces long carbon chain polymer fragments, which compensate for the decrease in viscosity and thus extend the service life of the lubricant.
In the course of their work the researchers produced macrocyclic polymers – primarily polypentenamers – with silicon-supported, ruthenium-based olefin metathesis catalysts, through the use of ring expansion metathesis polymerization (REMP). The results are protected by a US patent (Tuba, R.; Grubbs, R.H. US Patent App. 10,619,003).
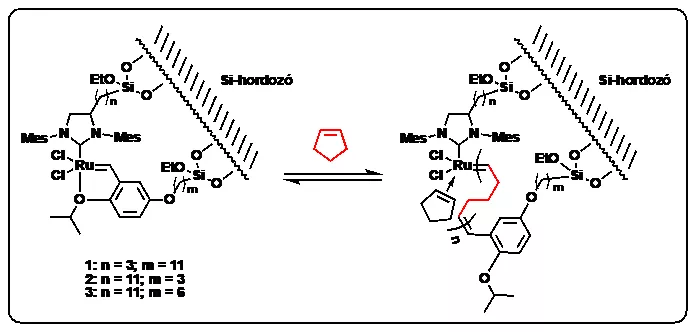
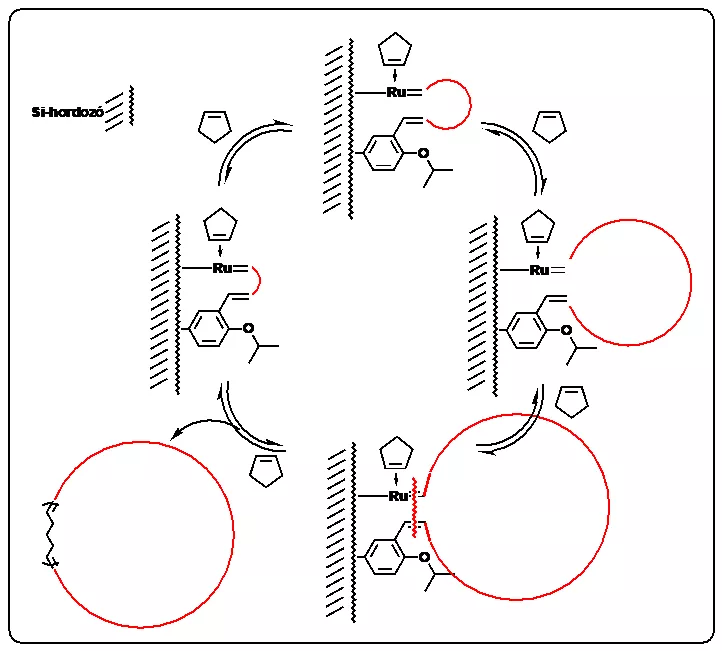
Synthesis of macrocyclic polymers through the ring-expansion metathesis polymerization (REMP) process
The literature considers polypentenemers to be a synthetic rubber base material, the physical properties of which are similar to those of natural rubber. It is generally believed that linear polymers, through their end groups, contribute to the aging of the polymers in tires, significantly reducing their performance and durability. Ring polymers are thus expected to alleviate these problems and improve the performance of tires and increase their service life.
It is also of great importance from the point of view of environmental protection that polypentenamers – regardless of whether they are linear or macrocyclic – can be produced by means of equilibrium olefin metathesis polymerization. This also includes the possibility that not only can these polymers be built up from cyclopentene with the given catalyst systems, but they can also be catalytically broken down into their starting components by slightly changing the reaction conditions. Careful design based on the research could create the possibility of producing high-performance tires that can be broken down into their original components after use and recycled.
The catalyst systems presented in this study show outstanding activity under mild reaction conditions. This is well exemplified by the fact that a single carrier-linked catalyst molecule can incorporate 415,000 monomers (TON > 415,000). Thanks to the heterogenization of the catalyst, the ruthenium contamination of the product is negligible, and the catalyst can also be recycled. These properties go far beyond today's expectations for sustainable catalysis.