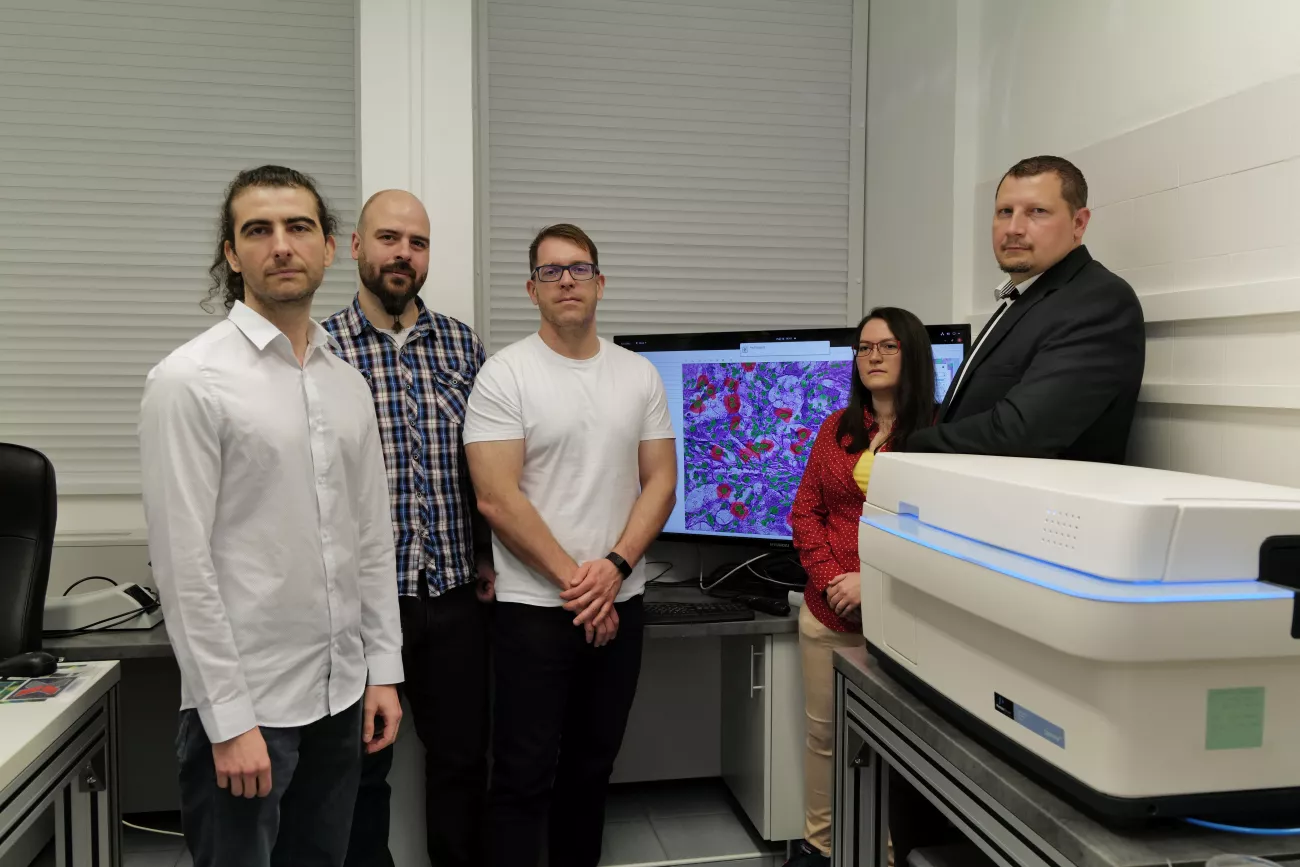
The research team of the ELKH Biological Research Centre (BRC) in Szeged, led by Dr Péter Horváth, has developed a novel artificial intelligence-based Deep Visual Proteomics image processing method in an international collaboration. The essence of the method is that it can describe abnormal protein changes within cells with extreme accuracy, which can contribute to the early diagnosis of cancer and the development of targeted treatments, among other things. The results have been published in the leading journal Nature Biotechnology.
The potential of artificial intelligence-driven microscopic image analysis for cellular analysis is of undisputed biomedical importance. By developing this technique to the level of analyzing proteins that determine the functionality of individual cells, invaluable information can be extracted for diagnostic and therapeutic purposes. For many years, Dr Péter Horváth, Director of the Institute of Biochemistry at the University of Szeged, and his research group have achieved outstanding results in microscopic image processing using artificial intelligence. Today, even the most important partners collaborating in this field rely heavily on the expertise of Szeged researchers. Single-cell analysis for the precise characterization of individual cells that make up tissues has become a routine procedure in the Szeged laboratory, which delivers significant added value in systems biology and medical research thanks to a methodology that also allows the extraction of pathological cells in samples.

The researchers have now combined an image processing and cell retrieval method that can recognise individual cells with an ultra-sensitive single-cell proteomics technique that can describe pathological protein changes within cells with extreme accuracy, in addition to morphological abnormalities in pathological cells. The analysis of the cell’s total protein content provides key information for a better understanding of physiological and abnormal cellular functions, and thus has a major role to play in the detailed characterization of cancer processes, among other things, and thus in the further development of early diagnosis and targeted treatment, the authors of a recent publication on the Nature Biotechnology website point out, highlighting the practical potential of the new findings.
Operating principle of new method: search, excise, analyze
The operating principle of the special microscope system developed by researchers in Szeged is seemingly simple, but the method is capable of unusual functions. Cell recognition algorithms using a new branch of artificial intelligence called deep learning – in which, for the first time in the world, one artificial intelligence teaches another artificial intelligence – can recognize abnormal cells in digital images of tissue samples millions of times faster than the human eye and with equal or greater precision. This process is called phenotyping, which involves the isolation of individual cells from human tissues based on external characteristics. No matter how complex the sample, the algorithm will not miss even the smallest, cellular-level discrepancy.
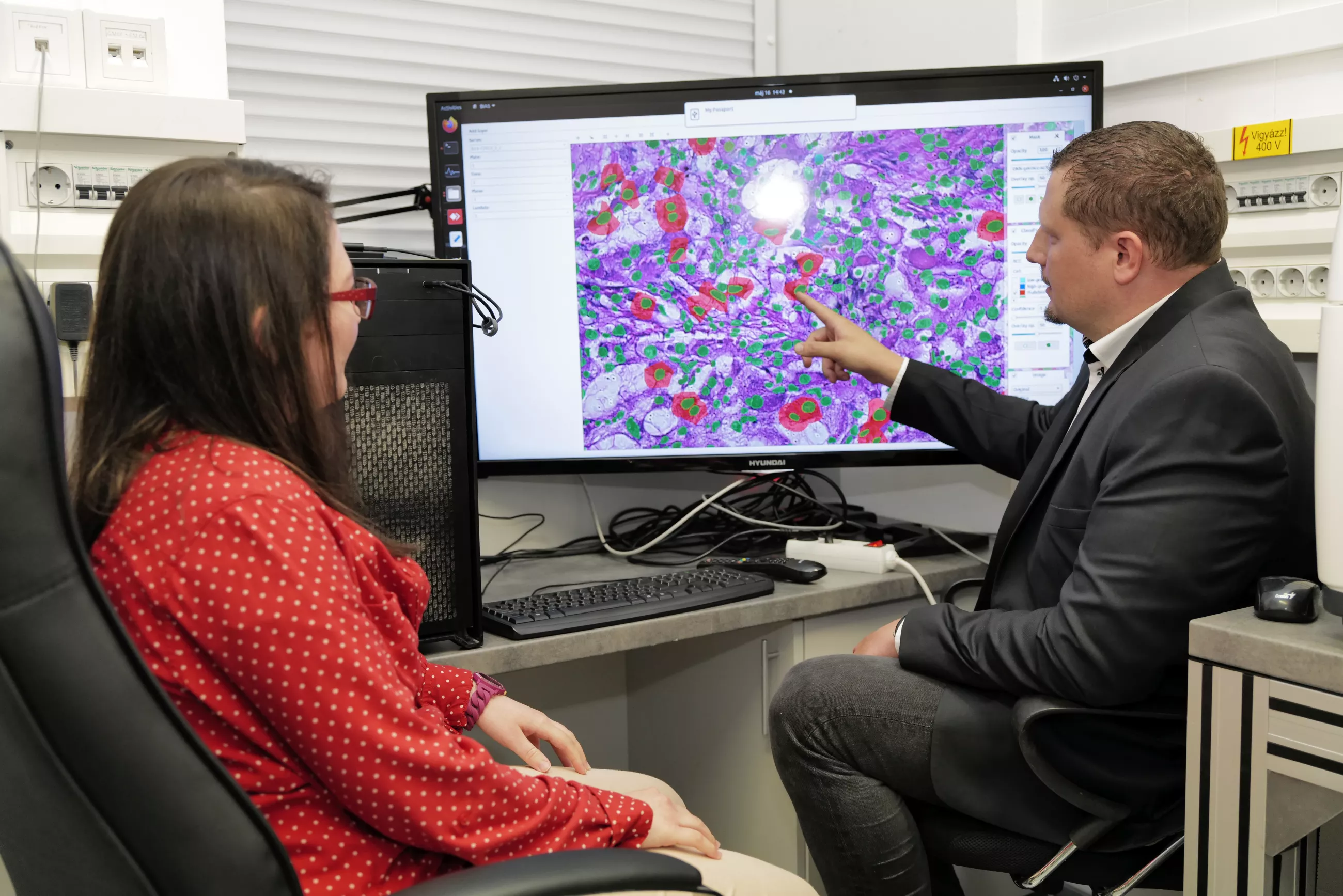
The pathological cells thus identified are excised from the sample by a laser microdissection microscope with a precision of one micrometer, followed by molecular analysis of the extracted single cells. The accuracy of a microscope performing cell excision is amply illustrated by the fact that the possible error of the excision following the cell’s profile is less than one hundredth of the thickness of a hair. In other words, this microscope would be able to engrave anyone’s family tree into a hundred micrometer-thick strand of hair.
Until now, molecular analysis of excised individual cells has essentially focused on describing the cell’s DNA make-up – for example, to detect tumour mutations – which, although a huge step forward in personalized therapy, provides limited information on the actual functionality of the pathological cells, which is determined by the proteins produced in the cells.
Developing new methods through collaborations of excellence
Dr Péter Horváth and his research team launched a joint research project around two years ago with Matthias Mann, considered the father of proteomics, and Emma Lundberg, also known as one of the creators of the Human Protein Atlas. Matthias Mann is Europe’s most cited researcher, Head of the Institute of Biochemistry at the Max Planck Institute in Munich and Professor at the University of Copenhagen. Emma Lundberg is a professor at Stanford University and the Royal Institute of Technology, Sweden, and one of the most cited young researchers in the field of proteomics and cell biology. In a major project supported by the European Union and the Zuckerberg Foundation (Chan Zuckerberg Initiative), they have developed a molecular method that miniaturizes the mass spectrometric analysis of proteins down to the single-cell level. By increasing the sensitivity of the analysis by a factor of around 100, it is now possible to quantify thousands of proteins in a single sample, giving a precise picture of the cell’s protein stock. But the work does not stop there, as researchers at Stanford University are also investigating the biological role of proteins that appear to be unique to pathological cells, which could lead to the identification of new therapeutic targets in the near future.
The innovative technology development is closely linked to the Human Cell Atlas project, also supported by the Chan Zuckerberg Initiative, which is currently one of the largest biological research projects in the world. Its aim is to characterize each cell type in the human body as accurately as possible, and in doing so, to map in minute detail the relationship between different cell types, the connections between the cells that make up tissues, the interaction between organs and organ systems, and ultimately, how changes in the three-dimensional map affect the health of individual organs and the body as a whole.
New perspectives in cancer diagnostics and treatment
Even a single abnormality in one of the many billions of cells in the body is enough to trigger irreversible processes – think of different types of leukaemia triggered by a mutation in a bone marrow stem cell. Finding these initial pathological cells can be a key tool for cancer prevention and early treatment. Given that modern oncology drugs typically work by targeting a pathological protein function of some kind, mapping protein changes in abnormal cells could give a new impetus to the development of oncotherapies.
According to Dr Péter Horváth, single-cell proteomics based on artificial intelligence could enter clinical practice in the next two years and bring a breakthrough in personalized oncotherapy. This vision will be supported by the Thematic Center of Excellence, currently under construction in Szeged, which is intended to become the world’s most advanced single-cell research and analysis center.