An international consortium of Hungarian, American and Italian research groups, led by the Biomolecular Interactions Research Group of the ELKH Research Centre for Natural Sciences (TTK), has studied how a herpesvirus protein alters the function of enzymes that regulate host cell division. Among other things, the researchers highlighted the weaknesses of cellular signaling processes and discovered new ways to reduce the growth disorders of human cells. A study presenting the results of the research has been published in the journal Nature Communications.
The disease sarcoma, first described and named after Mór Kaposi in 1872, was found at the end of the last century to be linked to herpesvirus infection at the end of the last century. Herpesviruses are DNA viruses that have long developed in humans and no longer cause serious illness in healthy people. That is why the researchers assumed that such viruses had thoroughly learned how the host works, so that they could modify it in a way that suited them. In this way, it could be used as a model in a 'tamed' form.
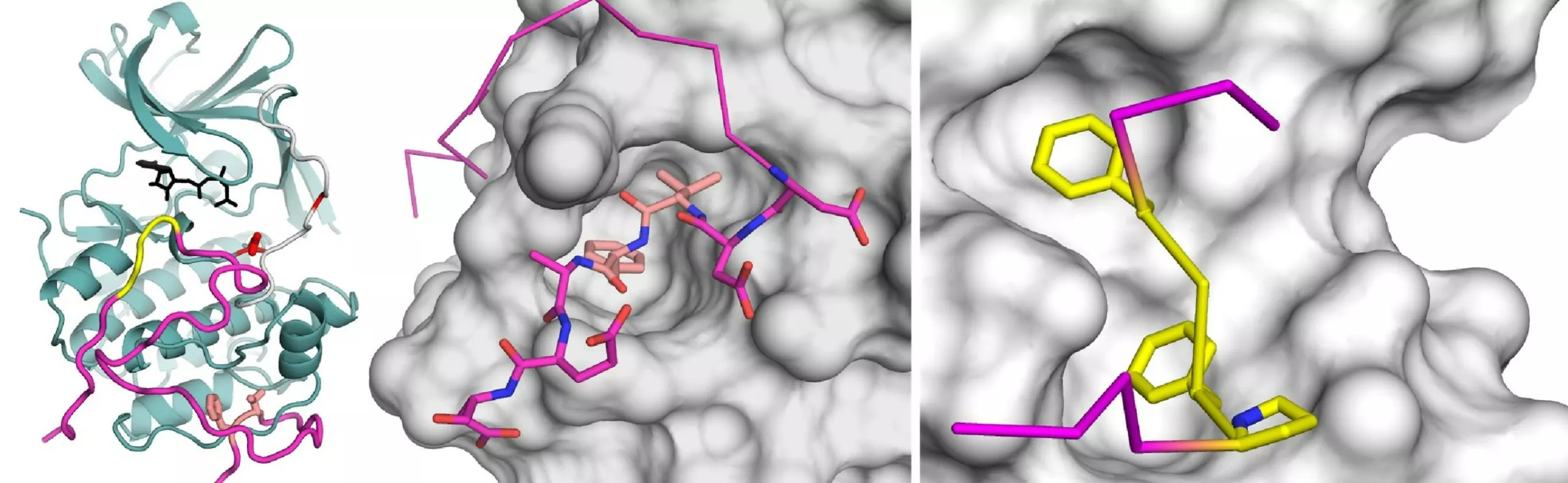
Using biochemical methods and computer simulations, the international consortium studied the effect of one of the proteins of Kaposi's sarcoma-associated herpesvirus (KSHV) (ORF45) on human cells. It was established that the KSHV ORF45 protein simultaneously binds two key protein kinases of important signaling pathways in cell growth, thereby altering the dynamics of the formation and separation of natural complexes within the host cell, which in turn leads to increased cell growth signaling activity. Because kinases are enzymes that alter the function of other proteins through phosphorylation, i.e., a common chemical protein modification mechanism, the phosphorylation of a group of proteins is increased in the presence of the viral protein.
The research team also made atomic-resolution images of the complexes formed by the two enzymes and the viral protein regions that specialize in binding them. The results not only explained how the viral protein reprograms the function of human protein complexes, but the researchers also uncovered unknown protein-protein interactions that are also required for the proper functioning of the host cell.
Herpesvirus proteins are grand masters of molecular-level disturbances that cause pathological functions in host cells. Like other viruses, Kaposi's sarcoma-associated herpesvirus has 'recognized' weaknesses in complex intracellular regulatory systems. The specific mechanism is based on molecular mimicrine, in which the viral protein binds to protein surfaces located on enzymes responsible for regulating the delicate balance of cell growth and cell death.
Understanding the biochemical tricks used by 'gentle viruses' offers further opportunities to curb the growth disorders of human cells – such as increased cell division, cancer or increased cell death, and inflammation. The research team is now developing compounds that bind to proteins that are also favored by the viral proteins that manipulate cell growth.
The research was supported by the NKFIH Leadership and the VEKOP-2.3.3-15-2016-00011 tender.