There is an increasingly strong emphasis on mapping the unique properties of cells in life science research. This is extremely important because the unique characteristics of the building blocks of various organs and tissues provide important information for better understanding the background of malfunctions and the earliest possible detection of disease processes. Researchers at the ELKH Szeged Biological Research Centre and the University of Szeged have developed a unique new method for studying the physiological function of brain cells. With their proprietary, artificial intelligence-controlled, automated microscope system, they are able to find any cell within a living tissue sample, stimulate cells with predefined characteristics, and collect biological information by recording response processes. The developed new method may open up new perspectives in the early diagnosis and understanding of such worldwide spread diseases as Alzheimer's or Parkinson's disease, thus supporting the development of effective therapies.
The Biological Image Processing and Machine Learning Working Group of the ELKH Szeged Biological Research Centre led by bioinformatician Dr Péter Horváth, and Dr Gábor Tamás, professor of neurobiology, head of the Cerebral Neural Networks Research Group at the University of Szeged, have been working together for several years on system microscopy solutions that have opened new avenues for the study of individual cells. Their latest development is the Autopatcher, an electrophysiological procedure using artificial intelligence, which was featured in the highly prestigious journal Nature Communications on February 10. The method is unique in several respects: cell tests are performed on native (unstained or otherwise unlabeled) brain tissue samples using machine vision and artificial intelligence. Using deep learning algorithms, the software, based on the analysis of thousands of images, is able to automatically determine the location of the micropipette integrated into the microscope based on the camera image and precisely move the pipette, automatically detecting target cells and their spatial displacement. Depending on the purpose of the test, the artificial intelligence-controlled system selects each target cell to ensure that the success of the measurement is as high as possible. The new technology will contribute, among others, to the discovery of new human cell types or a better understanding of the connections of brain neurons. Professor Gábor Tamás had previously discovered a new type of human brain cell in a similar way, and the new method just developed also anticipates further discoveries of great significance.
Machine vision and automation
Machine learning – i.e. artificial intelligence, or one of its varieties called deep learning – algorithms based on the images of the camera built into the microscope system control the micropipette. Machine vision provides much more precise targeting, measured in micrometers, than the human eye. After the machine-learning phase, the system is already able to detect specific cell types in an unknown brain tissue sample. A miniature electrode built into a pipette directed to the membrane of the selected cell is capable of individually stimulating the cells. By following the response to finely controlled stimulation, important information about physiological cell activity can be obtained without damaging the cell. In other cases, using the air pressure control system built into the pipette, it is possible to remove even the nucleus and the cytoplasm, and carry out molecular single-cell analysis on this basis, which can be an important source of genetic information, for example in combination with gene sequencing, explains Krisztián Koós, the first author of a paper published by the research group. The research and the development of the microscope system have great long-term potential: they can put drug trials on a new footing, for example, by allowing cell-level drug effects to be traced on a living tissue sample. By installing another micropipette, the system can make it possible to investigate the connections between neurons: to determine the characteristics of the cellular response to stimulation, and analyze the influence of stimulus propagation.
Unlabeled, precise cell analysis
Label-free examination of cells is another important innovation of the development project carried out in Szeged. Staining methods widely used to identify cell types (e.g. fluorescent staining) necessarily result in cell death in living tissues, so studies of cell function are precluded. Although there are other, less drastic cell labeling methods (e.g. genetically modified fluorescent cell labeling methods), their use is not feasible for many cell types. This means that native (unstained) cell testing overcomes a number of disadvantages that have so far hampered the testing of individual cells.
The operation of an automated system microscope is clearly illustrated in this video.
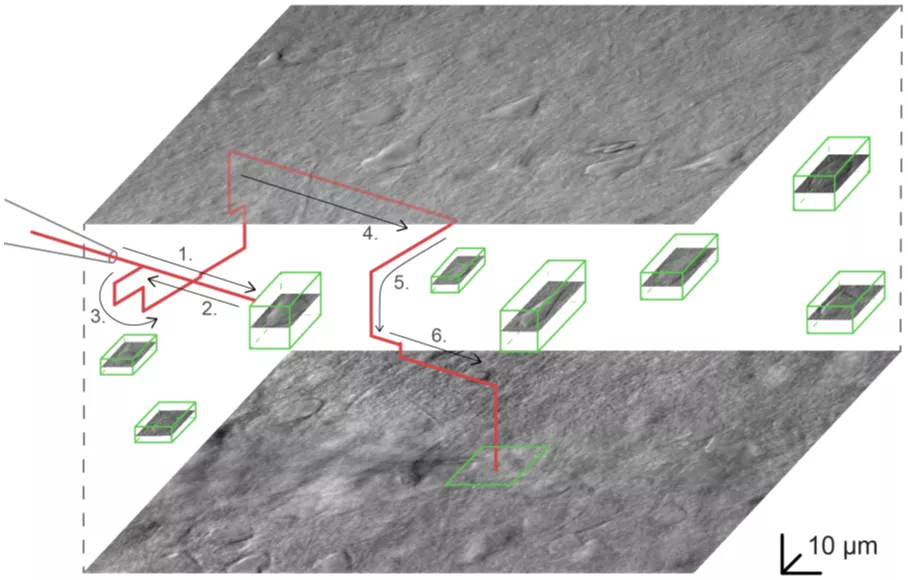
The system plans the path of the pipette to approach the target cell and avoids any obstructions. (Source: Nature Communications)